Healthy Aging, Memory decline & Risk for
Alzheimer’s disease
Background
Memory, Emotion, and Brain Aging: A Lifespan Approach
The global population is increasingly aging, thus maintaining healthy brain aging and prevention of Alzheimer’s disease (AD) are some of the most important public health/ economic challenges of our time. The CNL-SD studies aging using a lifespan perspective beginning in fetal development to explore developmental antecedents to brain aging, with a specific focus on key periods of sexual differentiation. Primary foci include 2 brain circuitries particularly vulnerable in early aging: memory and emotion circuitries. We take an innovative approach by integrating multi-modal imaging methods with early biomarkers and novel genetic strategies for understanding sex differences in brain aging and risk for AD.
Disinhibition of emotion regulation impacts memory and other cognitive functions in a sex-dependent way.
Impact of Sex Differences on Brain Aging
Two thirds of AD patients are women. There are sex-dependent brain and physiologic factors that contribute to higher frequency of AD in women, over and above longevity. Reproductive aging (i.e., menopause) is a critical period for women. Estradiol (that declines with menopause) has neuroprotective, anti-inflammatory, and antioxidant properties, and thus, menopause is a window of vulnerability increasing susceptibility to neurodegeneration and cognitive impairment. By following women in midlife as they transition through menopause, our group has been identifying the impact of sex steroid hormone changes on sex differences in memory circuitry decline and AD in later life.
We are also studying early antecedents to sex differences in decline in emotion regulation with age and the role of reproductive age as distinct from chronologic age. Disinhibition of emotion regulation impacts memory and other cognitive functions in a sex-dependent way.
A Sample of Key Findings.
One.
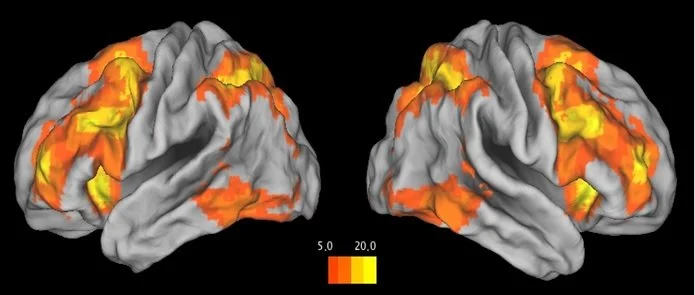
Task-evoked responses in working memory circuitry (dorsolateral prefrontal cortex & hippocampus) were altered as a function of women’s reproductive stage.
With advancing reproductive age, there was a shift in network reliance (from PFC-parietal to PFC-hippocampal pathways) for successful working memory performance.
Changes in working memory function were strongly related to a decline in estradiol.
Two.
Task-evoked response in hippocampus decreased over the menopausal transition and correlated with decreasing levels of estradiol.
Postmenopausal women showed enhanced bilateral hippocampal connectivity relative to premenopausal and perimenopausal women to maintain successful verbal encoding.
Three.
Women outperformed men on verbal and associative memory tasks.
Female advantage in verbal memory was attenuated postmenopause due, in part, to estradiol decline.
Four.
In postmenopausal women, lower levels of BDNF were associated with worse memory performance and altered function in the working memory circuitry.
BDNF may modulate the impact of ovarian function decline on memory in early midlife.
Five.
Lower levels of DHEAS were associated with altered memory circuitry function, specifically in postmenopausal women with major depression.
In women, major depression and inability of adrenal gland to provide estrogenicity after menopause may contribute to a lower ability to maintain intact memory function with age.
Highlighted Works.
Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife
Sex differences in episodic memory in early midlife: Impact of reproductive aging
Impact of BDNF and sex on maintaining intact memory function in early midlife
Prenatal Origins of Brain Aging
Growing evidence for a fetal antecedence to older adult memory impairments has implicated disruption of prenatal stress and immune pathways. Using an unprecedented prenatal longitudinal cohort (see website section on “Study Populations”), we are linking fetal exposures to sex differences in memory decline and amyloid deposition 60+ years later. We have deeply characterized these offspring with regard to brain and physiology phenotyping coupled with genetic and transcriptomic analyses that we couple with developmental risks and exposures.
A Sample of Key Findings
One.
Abnormalities in prenatal maternal immune activation during critical periods of the sexual differentiation of the brain produced long-lasting effects on memory circuitry/function from childhood to midlife, that were sex-dependent, brain region-specific, and, within women, reproductive stage-dependent.
Two.
Preeclampsia and fetal growth restriction impact memory circuitry and function by sex and reproductive stage 60 years later.
Development of a Clinical Risk Algorithm for prediction of AD in Early Midlife
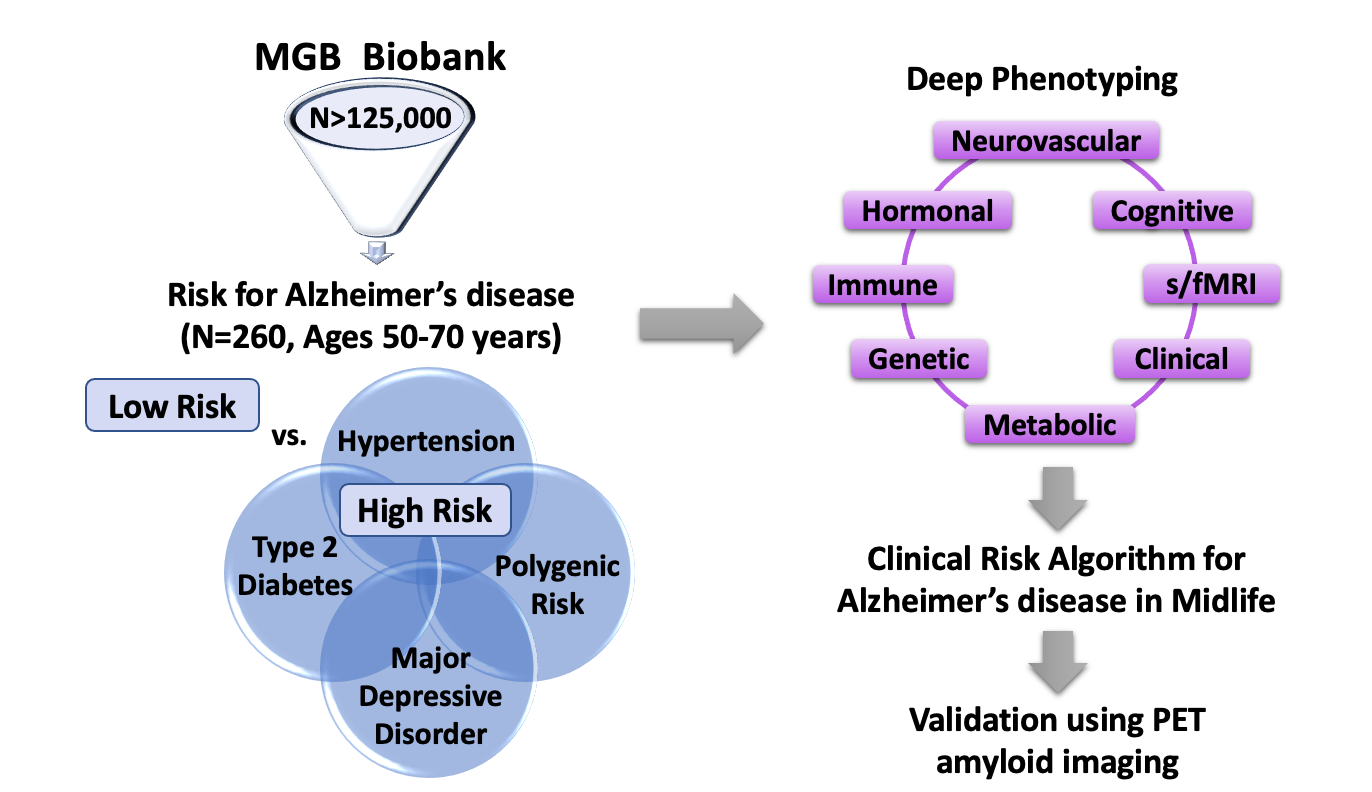
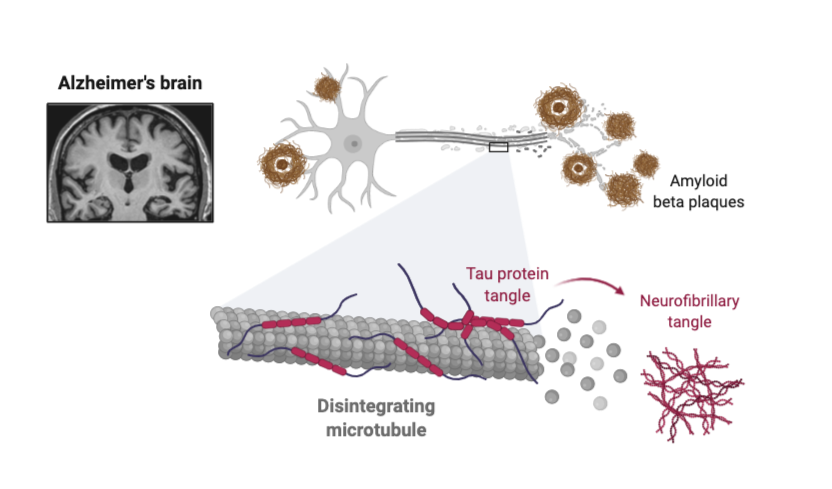
Although late onset AD occurs at ages 65+, accumulation of pathology occurs 15-20 years prior to diagnosis. Secondary to genetics, cardiometabolic diseases (CMD) (e.g., hypertension and diabetes) in midlife are independent risk factors for AD. Despite significant sex differences in pathology, timing, and clinical presentation in CMD and AD, shared pathophysiology underlying CMD and AD, and sex differences therein, are largely unexplored.
For early prevention, it is critical to develop sex-dependent risk assessments to identify in earlier midlife those at highest risk for AD later in life, prior to disease manifestation. We are leveraging the MassGeneral Biobank to create a Healthy Aging Translational CoHort (HATCH), composed of individuals between ages 50 – 70 years who are high- and low-risk for AD. High-risk is defined as genetic risk and/or clinical risk (i.e., having a diagnosis of either major depression, hypertension, and/or diabetes, known major risk factors for AD). Low-risk individuals do not have any of these. We are characterizing high- and low-risk individuals extensively assessing genetic, immune, hormonal, neurovascular, metabolic, and clinical functions. Using HATCH, we aim to develop a clinical risk algorithm that will identify in early midlife those who are at highest risk for cognitive decline and AD later in life.