Sex Differences
in Psychoses
Background
For more than 30 years, Dr. Goldstein and her team have investigated sex differences in schizophrenia, i.e., why men are at higher risk than women.
Her lab conducted the most extensive brain imaging work on this topic in the late 1990’s, hypothesizing sex differences in schizophrenia beginning in fetal development during critical periods of the sexual differentiation of the brain.
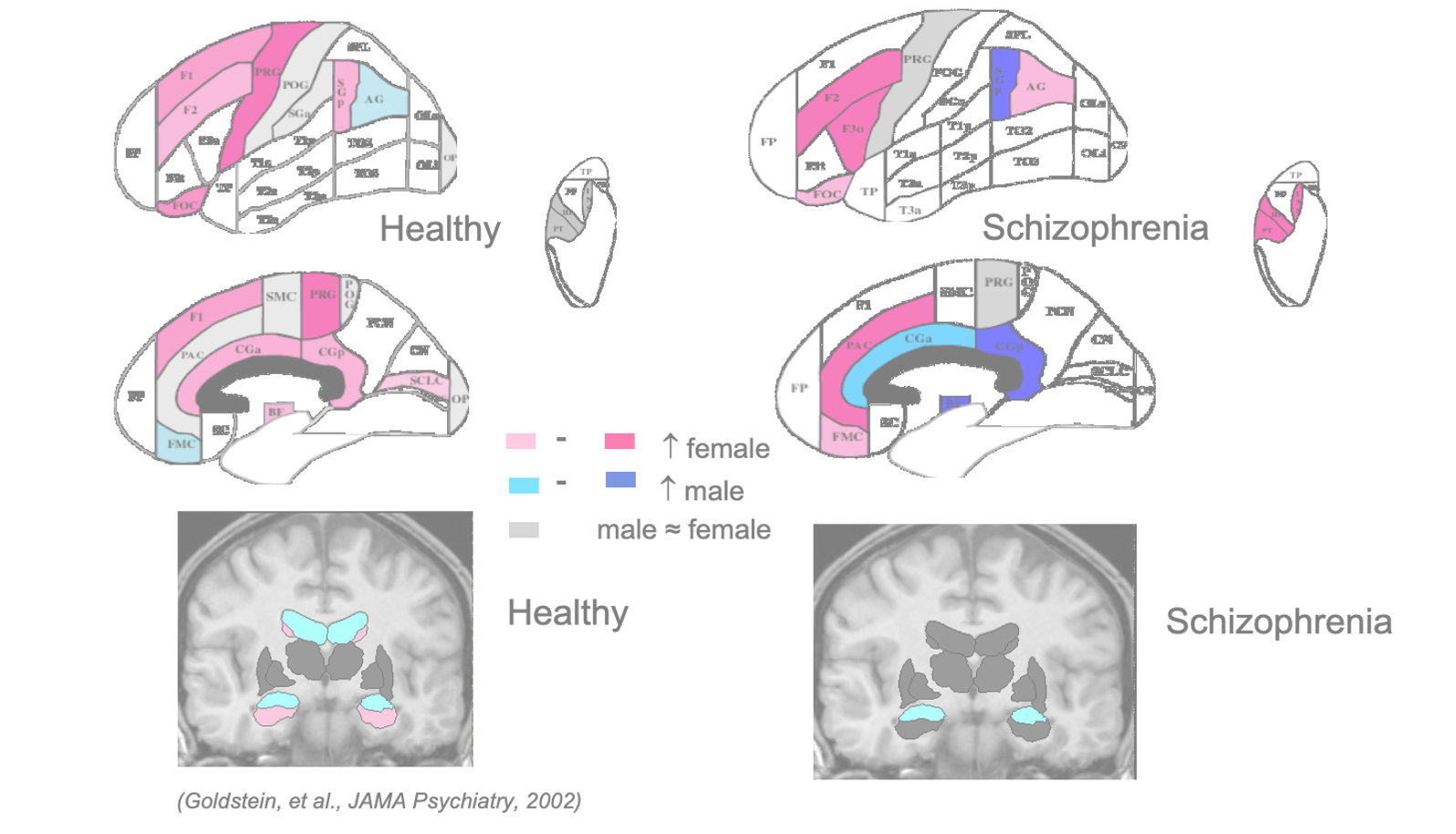
Early work also characterized sex differences in symptomatology, premorbid history/age at onset, course and treatment responses, genetic transmission, and incidence. Subsequent functional and structural imaging studies mapped out sex differences in brain abnormalities including verbal memory circuitry. In 2011, using structural MRI, we showed significant sex differences in memory circuitry in schizophrenia that differed from sex differences in memory circuitry the healthy brain. (Abbs, et al. 2011).
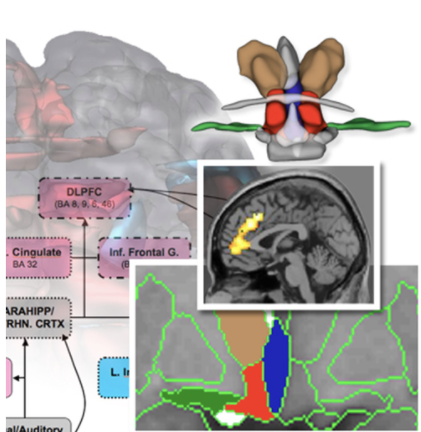
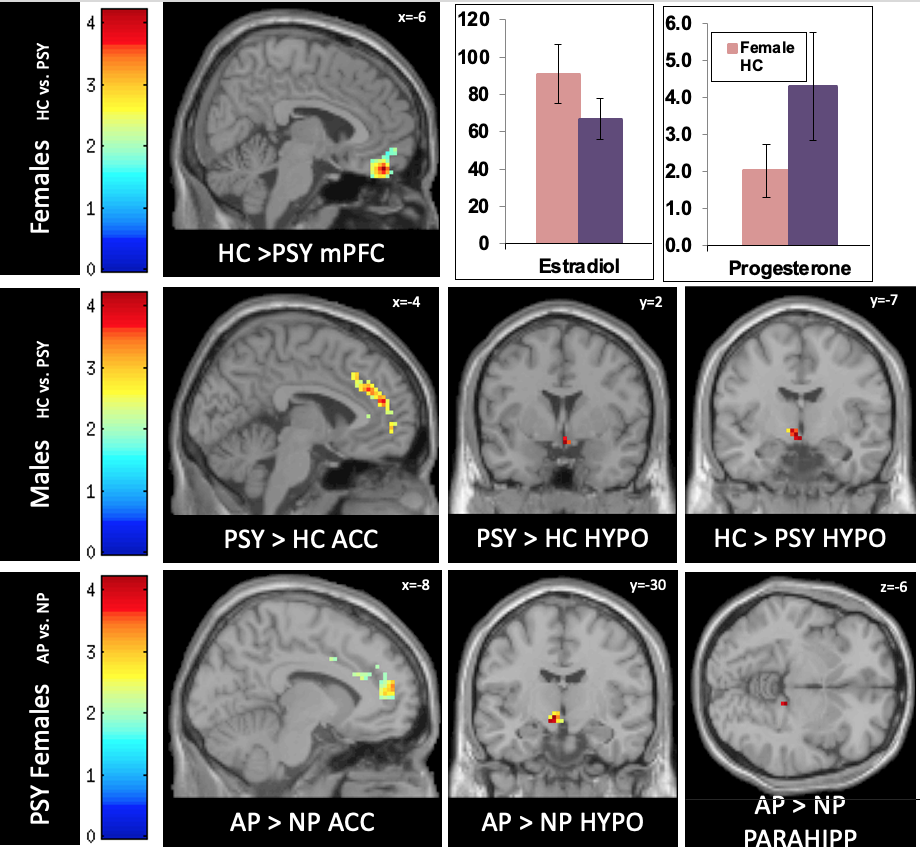
Functional MRI studies focused on stress and memory circuitries in psychoses. Stress is associated with every psychiatric disorder. In the service of understanding shared pathophysiology, using fMRI we demonstrated significant sex differences in dysregulation of stress response circuitry that was shared across 3 psychiatric disorders (depression, schizophrenia, and bipolar disorder) that was dependent on depressed mood and associated with steroid hormones. (Marečková, et al, Hum Brain Mapp 2016).
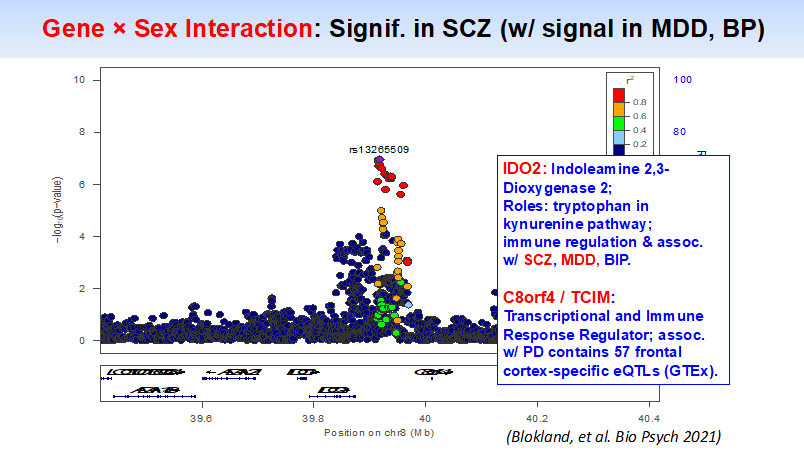
Further, using “big data” in the largest sex by genotype study to date (2022), we demonstrated significant sex differences in genes associated with depression, schizophrenia, and bipolar disorder. (Blokland, et al, 2021)
“Sex-by-genotype differences, shared across depression and psychoses, include neuronal, vascular & immune pathways”
Finally, our team has been mapping out the early prenatal antecedents to the sex differences in the brain in psychoses that we identified. (e.g. above examples). We have focused on maternal-to-fetal transmission of steroid hormone, genes, and immune dysregulation that disrupt the healthy sexual differentiation of the brain resulting in sex differential vulnerability for risk for schizophrenia, sex-dependent brain abnormalities in stress response and memory circuitries, and comorbid neuroendocrine and ANS dysfunction. Studies were conducted with the adult offspring of the prenatal cohort (described elsewhere on the site), including multi-modal imaging (i.e., sMRI/fMRI/DTI) in tandem with blood collection for immune and steroid hormone physiology and genetic/genomics to test hypotheses related to fetal programming. (Goldstein, et al. PNAS. 2021)
Schizophrenia work (1988 - 2015)
Gender differences in the expression of schizophrenia
Gender differences in the course of schizophrenia
Sex differences in the familial transmission of schizophrenia
Genetic heterogeneity may in part explain sex differences in the familial risk for schizophrenia
Are there sex differences in neuropsychological functions among patients with schizophrenia?
Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging
Prenatal maternal immune disruption and sex-dependent risk for psychoses